Structure + Content
In this CAS, you gain strategic knowledge. Knowledge, that involves communication and negotiations with stakeholders and authorities, process optimization through AI, sustainable regulatory strategies, and effective interface management.
Portrait

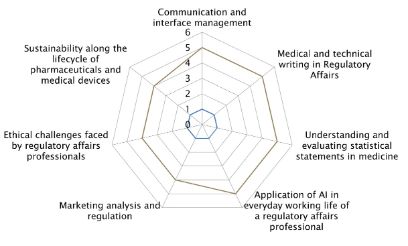
This programme emphasises communication (speaking and writing) as a critical skill, particularly for regulatory professionals who interact with a global audience, including manufacturers, authorities, and Notified Bodies.
To develop clinical trials, an understanding of statistical methods and data interpretation is essential. You will learn the language of statistics and what questions to ask the statistician when you need to write your documents.
The integration of artificial intelligence (AI) is transforming the regulatory landscape. This CAS explores how AI can streamline processes.
To engage effectively with Supply Chain 4.0, an understanding of technologies such as artificial intelligence (AI), the internet of things (IoT), and data analytics are essential. You will explore how these tools drive real-time visibility, automation, and faster decision-making, enhancing responsiveness, efficiency and patient safety.
This CAS addresses also the ethical und sustainability challenges in regulatory affairs, including their impact on clinical trials, product development, and environmental compliance.