Structure + Content
In this CAS, you achieve the skills to work in a highly regulated environment.
You achieve the skills to work in a highly regulated environment and learn how to act as a liaison person between companies and regulatory authorities.
This CAS:

Start your continuing education on 24 April 2026.
In this CAS, you achieve the skills to work in a highly regulated environment.
The individual modules that make up this programme are named below.
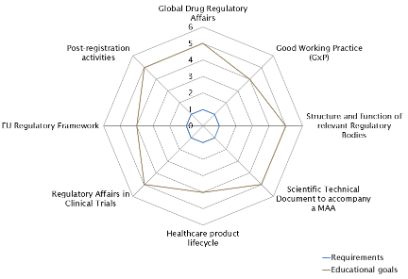
A module may include a variety of teaching methods such as lectures, seminars, case studies, practical labs, assignments, etc.
For the 12 ECTS credits to be recognized, successful completion of the competency assessment is required (exams, semester work).

The continuing education program in Regulatory Affairs in Pharma and Life Sciences provides an excellent foundation for understanding global regulatory frameworks. It is helping me strengthen my expertise in regulatory affairs and bridge my prior experience in pharmacovigilance with new regulatory knowledge. It offers a perfect balance of academic and real-world learning.

The CAS in Regulatory Affairs Pharma provided me with a comprehensive understanding of the regulatory landscape and practical tools for daily work in the pharmaceutical industry. I especially valued the combination of expert lecturers and real-world case studies, which made the learning experience both relevant and applicable.

The CAS has a compact design and provides an excellent overview of RA in the EU, US and Switzerland. The presentations given by colleagues from the field and the Swissmedic enabled a valuable exchange of ideas, combining theory with practice. The knowledge I acquired is very helpful in my professional life going forward. The wonderful interaction with my fellow students, coupled with the relaxed and friendly atmosphere, made this CAS a truly special experience.
Certificate of Advanced Studies (CAS) in «Regulatory Affairs Pharma»

Find out which professionals this continuing education program is designed for and what requirements you need to bring with you.
Ideally you have a medical, pharmacy or science degree, an engineering education, legal studies, a higher education in material science, a degree in Business Administration or in a related field.
As a rule, a university degree and practical experience are required for admission.
People with a high vocational training can apply, provided they have suitable professional experience and prior scientific and methodological knowledge.

Do you have questions about the programme? The head degree of programme, CAS heads, and lecturers will answer your questions at our information events.

Please refer to the pages for the respective degree programmes (CAS, SAS) for information on how and where your continuing education will take place. We are located at Aarbergstrasse 46 in the Switzerland Innovation Park Biel/Bienne.
Biel Aarbergstrasse 46 (Switzerland Innovation Park Biel/Bienne)